Describe the Difference Between Heat and Temperature
Whats the difference between heat and temperature. Unlike temperature heat is the transfer of energy from a hotter body to a colder body.
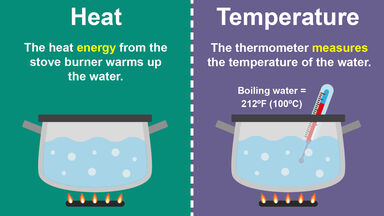
Difference Between Heat And Temperature In Simple Terms
Specifically temperature is a direct measure of the average kinetic energy of the particles in an object.

. There are three types of heat flow in substances. Describe the difference between heat and temperature in terms of kinetic energy. 3 on a question.
Write a chemical equation showing an exothernic reaction. Heat is a one type of energy but Temperature is intensity of heat Simply temperature express intensity of heat. Describe the difference between heat and temperature Heat energy is the energy from CHEM 1331 at University of Houston.
Describe the difference between heat and temperature in terms of kinetic energy. Faster or slower movement of molecules is noticed depending upon the kinetic energy whereas for thermal energy the total number of particles present in an object determines its kinetic energy therefore the bigger the. Describe the difference between heat and temperature in terms of kinetic energy - 23669642 sophialw05 sophialw05 3 weeks ago Chemistry High School answered Describe the difference between heat and temperature in terms of kinetic energy 1 See answer sophialw05 is waiting for your help.
The burner is at a higher. How much heat is required to raise the temperature of 80. When making macaroni and cheese you boil water.
Describe the difference between heat and temperature. Conduction convection and radiation. Temperature is directly proportional to the heat of the body so when heat is introduced the temperature of the.
Difference Between Heat and Temperature Heat and Temperature are physical properties of a body. Conduction is the transfer of heat in solids. More heat usually means a higher.
Heat and temperature are two different quantities. Heat is the sum of all the kinetic energies of all the molecules of an object while temperature is the average kinetic energy of the molecules of an object. Leave a Reply Cancel reply.
The main difference between temperature and thermal energy is that the temperature is not determined by the quantity of the object. The temperature of an object is the measure of an objects average Kinetic Energy. Often the concepts of heat and temperature are thought to be the same but they are not Heat and Temperature para.
The main difference between specific heat and heat capacity is that specific heat is the amount of energy needed to raise the temperature of a given sample by 1 K while heat capacity is the amount of energy needed to raise the temperature of. Specific heat and heat capacity both describe an amount of energy needed to raise the temperature of a substance. Posted on May 22 2021 by hakan tokmak.
Temperature is related to as average kinetic energy of moles from the equipartition theorem. Temperature is a measure of how hot or cold an object is. Teacher swabs rubbing alcohol on the students back of hand.
There exist a fine line which demarcates heat from temperature in the sense that heat is. While heat is a form of energy temperature is a measure of how hot a body is. It is sometimes referred to as thermal energy.
Write a chemical equation showing an endothermic reaction. Lesson Plan for Heat Vs Temperature Prior Knowledge. I am not able to think or find any difference between the two.
6 rows Heat and temperature are related and often confused. Now we know as temperature of room increases heat of the room also increases. To observe and explain differences between temperature and heat Engage.
Heat is measured by calorimeter and. The energy that is transferred is called heat Example. When two objects of different temperatures come in contact with each other some of that energy is transferred to the object with the lower temperature.
All chemical reactions involve a change in heat. Students have studied kinetic energy in their kinematics unit Objective. Add your answer and earn points.
Describe the difference between heat and temperature in terms of kinetic energy. The basic difference between heat and. Heat and Temperature Heat is often described by the average individual as being the change in temperature from hot to cold.
Describe the difference between heat and temperature in terms of kinetic energy in progress 0 Chemistry Linh Đan 5 months 2021-08-07T1829290000 2021-08-07T1829290000 1 Answers 5 views 0. 9 rows The SI unit Heat is Joule and Temperature is Kelvin.

Difference Between Heat And Temperature In Tabular Form
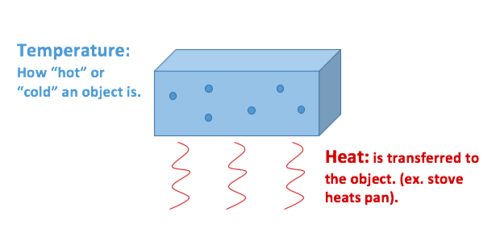
Heat Vs Temperature Energy Education
Difference Between Temperature And Thermal Energy Difference Between
No comments for "Describe the Difference Between Heat and Temperature"
Post a Comment